Projects Experimental Virology

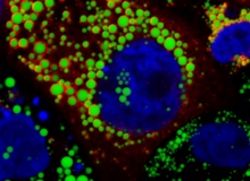
Cellular lipid droplets are important to initiate HCV assembly and facilitate interaction with lipoproteins. Lipid droplets were stained with a lipid dye (green color) and HCV proteins decorating lipid droplets were stained in red. Cellular nuclei were counterstained in blue (Dr. Gabrielle Vieyres).
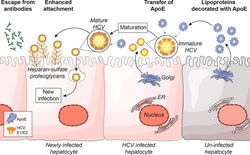
Model of HCV particle maturation after virion shedding by infected cells. This figure was published in Journal of Hepatology, Volume 67, Bankwitz et al., Maturation of secreted HCV particles by incorporation of secreted ApoE protects from antibodies by enhancing infectivity, 480-489, Copyright Elsevier (2017).
Fifty to 90 percent of all HCV infections are chronic. The association of the virus with lipoproteins contributes to its persistence by directly influencing virus entry into the liver cell and protecting it from neutralizing antibodies.
We are investigating host factors and viral factors that influence the association of the virus with lipoproteins to elucidate their role in viral persistence. The interaction of HCV with lipoproteins begins during virus assembly. This occurs in the so-called “lipid droplets”, cellular organelles that store lipids (Figure 1: Vieyres et al. PLoS Pathogens 2016). HCV uses cellular lipases such as ATGL to mobilize lipids for the formation of HCV lipoviroparticles (Vieyres et al. PLoS Pathogens 2020).
Following the release of HCV particles, circulating lipoproteins are incorporated into these particles (Bankwitz et al. J Hepatol 2017). This maturation process promotes viral attachment to liver cells and antibody protection (Figure 2 and Bankwitz et al. J Hepatol 2017). Supported by SFB900, we investigate the cellular and viral proteins that mediate the interaction between HCV and lipoproteins. Ultimately, the insights gained into how HCV escapes the antibody response with the help of lipoproteins will guide the development of a vaccine against HCV.
In a small number of HCV patients, strong immune responses lead to control and eventual elimination of the viral infection. However, the properties of this natural immune protection are not well understood. Most importantly, the role of antibodies in HCV protection is not clearly defined. Therefore, supported by the DZIF HCV vaccine project, we investigate how antibodies protect against HCV infection. What are the properties of the antibodies that are generated during HCV infection that heal? How do these antibodies differ from those produced when the infection becomes chronic? Are different areas of viral envelope proteins attacked? What factors influence the formation of particularly good antibodies, and how good are the antibodies that induce vaccine candidates? We are investigating these questions to develop a vaccine that induces particularly good protective antibodies.
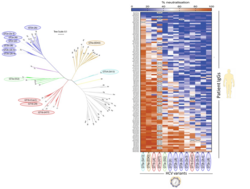
To develop a precise measure of protective immunity, we first isolated antibodies from the blood of 104 HCV-positive patients and then screened them for their neutralizing properties against as many different HCV variants as possible. Using bioinformatics, we learned that variants respond differently to antibodies, regardless of their genetic background. Our results may help develop a broadly effective vaccine in the future and assess the efficacy of vaccine candidates.
In collaboration with the Florian Klein Laboratory (Cologne), we recently used this system to isolate highly potent and broadly neutralizing antibodies from HCV patients (Weber et al. Immunity 2022). With funding from the DFG, DZIF, and the Helmholtz Association, we expand on this knowledge to develop HCV vaccine candidates.
Tour to HCV neutralization space. Final stop: B-cell vaccine | Nature Portfolio Webcasts
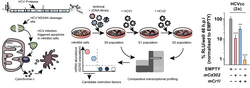
Using a cDNA library for genes expressed in the mouse liver, n4mBid cells were transduced and infected with HCV. n4mBid cells contain a modified Bid protein that is cleaved when the cell is productively infected with HCV, thereby forcing infected cells to die. Cells that contained protective genes survived and were thereby selected. Subsequent comparative gene expression analysis identified these protective genes. In this way, the lectin CD302 and the complement receptor CR1L were identified as restriction factors that individually and cooperatively impede HCV infection in liver cells.
Adapted from Brown et al. 2020.
Due to its narrow species tropism, natural infection of HCV only occurs in humans. The factors that determine this limited tropism are not yet fully understood. As a consequence, it is difficult to study HCV infection in animal models. This impedes the development of an HCV vaccine because it is impossible to directly evaluate whether a vaccine candidate can protect against an HCV infection in the absence of an immunological competent small animal model. The inability of HCV to replicate in non-human cells could be due to the absence or incompatibility of essential co-factors for viral replication and/or antiviral restriction mechanisms in non-human cells that effectively prevent viral replication. Under these assumptions, we apply various genetic screening systems to generate a comprehensive profile of all relevant factors for the species barrier to HCV infection in mouse cells. Using this information, we aim to develop in vivo models for HCV vaccine research. This project was funded by an ERC Starting Grant (VIRAFRONT, 2012-2017) and is now being pursued with support from the German Center for Infection Research (DZIF) and a grant from the National Institutes of Health (NIH, USA).Using a genome-wide cDNA screening, we identified two novel murine restriction factors that suppress HCV infection of murine liver cells. The lectin CD302 and the complement receptor CR1L cooperate, interfering with HCV cell entry and triggering transcriptional changes that block HCV infection (Brown et al. 2020). Knockout of CD302 increases the susceptibility to HCV infection of transgenic animals expressing the HCV receptor. These results reveal a new facet of liver-intrinsic immunity and point to new avenues for the development of animal models to further qualify HCV vaccines.
Interestingly, human CD302 is also expressed in human liver cells and partially restricts HCV and HEV infection (Reinecke et al. J. Virol. 2022).
We develop and use high-throughput screening assays to identify molecules with antiviral activity against RSV and SARS-CoV-2. For this purpose, we use luminescence- or reporter-based systems and make use of substance libraries of the HZI, the MHH and other external cooperation partners. For example, we cooperate with the Scripps Institute and use the ReFRAME repurposing compound library. The ReFRAME library is the world's largest collection of already approved or clinically proven compounds. The collections we use include a wide range of basic chemical structures, complex molecules from natural sources (secondary metabolites from bacteria, fungi, or plants), as well as already approved drugs. Our expertise in basic virological research enables us to elucidate the modes of action of these substances and viral resistance mechanisms. In doing so, we pursue the goal of developing new therapeutic options.
In collaboration with experts at the HZI, we have analyzed the mode of action of labyrinthopeptins against different viruses. Labyrinthopeptins bind certain lipids and lead to the destruction of the membranes of viruses. This makes them a very broad antiviral molecule, making it difficult for viruses to develop resistance to. (Blockus et al. 2020).
Funded by the Helmholtz International Laboratory for Anti-Infectives and as part of an international consortium, we have uncovered a new mode of action for treating RSV infection. RSV concentrates its components for RNA propagation in so-called "inclusion bodies". These fluid organelles play a crucial role in genome replication and viral transcription. The new compound hardens these viral replication organelles and disrupts, in this way, the infection of the virus (Risso-Ballester et al. Nature 2021).
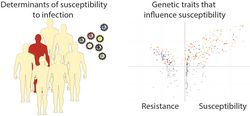
In some patients, an HCV infection will clear naturally, but in most cases, it becomes chronic. In addition, the course of chronic infection differs between patients. Most children are infected with RSV in the first 18 months of life. A fraction of these children suffer a severe course of the infection. For both conditions, the principles of underlying susceptibility and severity of infection are incompletely elucidated. However, this information is critical to ensure optimal prevention and medical care. For this reason, we are collaborating with our clinical partners at MHH to explore the principles of host susceptibility to these viral infections. Next generation sequencing technologies are utilized to examine host and pathogen characteristics to determine what role host genetics play in susceptibility to infection. Through collaboration with clinical researchers, we have access to unique patient cohorts that allow us to study viral pathogenesis in humans. This project is funded by the RESIST Cluster of Excellence and the INDIRA network.
Publications
Principles of assembly and cell entry of hepatitis C virus (HCV) and their relevance to resistance to antibodies
Vieyres G, Reichert I, Carpentier A, Vondran FWR, Pietschmann T. The ATGL lipase cooperates with ABHD5 to mobilize lipids for hepatitis C virus assembly. PLoS Pathog. 2020 Jun 15;16(6):e1008554. doi: 10.1371/journal.ppat.1008554. PMID: 32542055; PMCID: PMC7316345.
Bankwitz D, Doepke M, Hueging K, Weller R, Bruening J, Behrendt P, Lee JY, Vondran FWR, Manns MP, Bartenschlager R, Pietschmann T. Maturation of secreted HCV particles by incorporation of secreted ApoE protects from antibodies by enhancing infectivity. J Hepatol. 2017 Apr 22. pii: S0168-8278(17)30251-9.
Vieyres G, Welsch K, Gerold G, Gentzsch J, Kahl S, Vondran FW, Kaderali L, Pietschmann T. ABHD5/CGI-58, the Chanarin-Dorfman Syndrome Protein, Mobilises Lipid Stores for Hepatitis C Virus Production. PLoS Pathog. 2016 Apr 28;12(4):e1005568.
Gerold G, Meissner F, Bruening J, Welsch K, Perin PM, Baumert TF, Vondran FW, Kaderali L, Marcotrigiano J, Khan AG, Mann M, Rice CM, Pietschmann T. Quantitative Proteomics Identifies Serum Response Factor Binding Protein 1 as a Host Factor for Hepatitis C Virus Entry. Cell Rep. 2015 Aug 4;12(5):864-78.
Hueging K, Doepke M, Vieyres G, Bankwitz D, Frentzen A, Doerrbecker J, Gumz F, Haid S, Wölk B, Kaderali L, Pietschmann T. Apolipoprotein E codetermines tissue tropism of hepatitis C virus and is crucial for viral cell-to-cell transmission by contributing to a postenvelopment step of assembly. J Virol. 2014 Feb;88(3):1433-46.
Gentzsch J, Brohm C, Steinmann E, Friesland M, Menzel N, Vieyres G, Perin PM, Frentzen A, Kaderali L, Pietschmann T. hepatitis c Virus p7 is critical for capsid assembly and envelopment. PLoS Pathog. 2013;9(5):e1003355.
Funding:
SFB900: Microbial Persistence and its Control
RESIST: Resolving Infection Susceptibility
Determinants of protective immunity in HCV: A gold standard for vaccine development
Weber, T., Potthoff, J., Bizu, S., Labuhn, M., Dold, L., Schoofs, T., Horning, M., Ercanoglu, M. S., Kreer, C., Gieselmann, L., Vanshylla, K., Langhans, B., Janicki, H., Ströh, L. J., Knops, E., Nierhoff, D., Spengler, U., Kaiser, R., Bjorkman, P. J., Krey, T., … Klein, F. (2022). Analysis of antibodies from HCV elite neutralizers identifies genetic determinants of broad neutralization. Immunity, 55(2), 341–354.e7
Bankwitz, D., Bahai, A., Labuhn, M., Doepke, M., Ginkel, C., Khera, T., Todt, D., Ströh, L. J., Dold, L., Klein, F., Klawonn, F., Krey, T., Behrendt, P., Cornberg, M., McHardy, A. C., & Pietschmann, T. (2020). Hepatitis C reference viruses highlight potent antibody responses and diverse viral functional interactions with neutralising antibodies. Gut, gutjnl-2020-321190. Advance online publication. https://doi.org/10.1136/gutjnl-2020-321190
Bartenschlager R, Baumert TF, Bukh J, Houghton M, Lemon SM, Lindenbach BD, Lohmann V, Moradpour D, Pietschmann T, Rice CM, Thimme R, Wakita T (2018) Critical challenges and emerging opportunities in hepatitis C virus research in an era of potent antiviral therapy: considerations for scientists and funding agencies. Virus Res. 248: 53-62.
Vasiliauskaite I, Owsianka A, England P, Khan AG, Cole S, Bankwitz D, Foung SKH, Pietschmann T, Marcotrigiano J, Rey FA, Patel AH, Krey T (2017) Conformational Flexibility in the Immunoglobulin-Like Domain of the Hepatitis C Virus Glycoprotein E2. MBio 8(3).
Bankwitz D, Doepke M, Hueging K, Weller R, Bruening J, Behrendt P, Lee JY, Vondran FWR, Manns MP, Bartenschlager R, Pietschmann T (2017) Maturation of secreted HCV particles by incorporation of secreted apoE protects from antibodies by enhancing infectivity. J Hepatol 67(3): 480-489.
Bankwitz D, Pietschmann T (2016) Hepatitis C virus plays hide and seek with neutralizing antibodies. Hepatology 64(6): 1840-1842.
Funding:
DFG: Development of germline-targeting HCV E2 immunogens to drive neutralizing antibody evolution
DZIF: Hepatitis C Control: Toward prophylaxis and identificatin of those in need of treatment TTU 05.821_00
HZI: MCMVaccine
SFB900: Microbial Persistence and its Control
RESIST: Resolving Infection Susceptibility
Mechanisms of HCV tissue and species tropism
Repressors of viral infection, WO2017/207725A1Repressors of viral infection, WO2017/207725A1
Reinecke, B., Frericks, N., Lauber, C., Dinkelborg, K., Matthaei, A., Vondran, F., Behrendt, P., Haid, S., Brown, R., & Pietschmann, T. (2022). The Human Liver-Expressed Lectin CD302 Restricts Hepatitis C Virus Infection. Journal of virology, 96(7), e0199521.
Brown RJP, Tegtmeyer B, Sheldon J, Khera T, Anggakusuma, Todt D, Vieyres G, Weller R, Joecks S, Zhang Y, Sake S, Bankwitz D, Welsch K, Ginkel C, Engelmann M, Gerold G, Steinmann E, Yuan Q, Ott M, Vondran FWR, Krey T, Ströh LJ, Miskey C, Ivics Z, Herder V, Baumgärtner W, Lauber C, Seifert M, Tarr AW, McClure CP, Randall G, Baktash Y, Ploss A, Thi VLD, Michailidis E, Saeed M, Verhoye L, Meuleman P, Goedecke N, Wirth D, Rice CM, Pietschmann T. Liver-expressed Cd302 and Cr1l limit hepatitis C virus cross-species transmission to mice. Sci Adv. (2020) Nov 4;6(45):eabd3233
von Schaewen M, Dorner M, Hueging K, Foquet L, Gerges S, Hrebikova G, Heller B, Bitzegeio J, Doerrbecker J, Horwitz JA, Gerold G, Suerbaum S, Rice CM, Meuleman P, Pietschmann T, Ploss A (2016) Expanding the Host Range of Hepatitis C Virus through Viral Adaptation. MBio 7(6)
Anggakusuma, Brown RJ, Banda DH, Todt D, Vieyres G, Steinmann E, Pietschmann T (2016) Hepacivirus NS3/4A Proteases Interfere with MAVS Signaling in both Their Cognate Animal Hosts and Humans: Implications for Zoonotic Transmission. J Virol 90(23): 10670-10681.
Frentzen A, Anggakusuma, Gurlevik E, Hueging K, Knocke S, Ginkel C, Brown RJ, Heim M, Dill MT, Kroger A, Kalinke U, Kaderali L, Kuehnel F, Pietschmann T (2014) Cell entry, efficient RNA replication, and production of infectious hepatitis C virus progeny in mouse liver-derived cells. Hepatology 59(1): 78-88.
Finanzierung:
NIH:HCV
RESIST: Resolving Infection Susceptibility
DZIF: DELPHI TTU 05.910
Antiviral agents against HCV, RSV and coronavirus
Labyrinthopeptins as anti-viral agents EPA16809653.5, pending
Diagnostics and therapy for human respiratory syncytial virus (hRSV) EP17195522.2, pending
Risso-Ballester, J., Galloux, M., Cao, J., Le Goffic, R., Hontonnou, F., Jobart-Malfait, A., Desquesnes, A., Sake, S. M., Haid, S., Du, M., Zhang, X., Zhang, H., Wang, Z., Rincheval, V., Zhang, Y., Pietschmann, T., Eléouët, J. F., Rameix-Welti, M. A., & Altmeyer, R. (2021). A condensate-hardening drug blocks RSV replication in vivo. Nature, 595(7868), 596–599. https://doi.org/10.1038/s41586-021-03703-z
Blockus S, Sake SM, Wetzke M, Grethe C, Graalmann T, Pils M, Le Goffic R, Galloux M, Prochnow H, Rox K, Huttel S, Rupcic Z, Wiegmann B, Dijkman R, Rameix-Welti MA, Eleouet JF, Duprex WP, Thiel V, Hansen G, Bronstrup M, Haid S, Pietschmann T (2020) Labyrinthopeptins as virolytic inhibitors of respiratory syncytial virus cell entry. Antiviral Res 177: 104774.
Behrendt P, Perin P, Menzel N, Banda D, Pfaender S, Alves MP, Thiel V, Meulemann P, Colpitts CC, Schang LM, Vondran FWR, Anggakusuma, Manns MP, Steinmann E, Pietschmann T (2017) Pentagalloylglucose, a highly bioavailable polyphenolic compound present in Cortex moutan, efficiently blocks hepatitis C virus entry. Antiviral Res 147: 19-28
Pietschmann T (2017) Clinically Approved Ion Channel Inhibitors Close Gates for Hepatitis C Virus and Open Doors for Drug Repurposing in Infectious Viral Diseases. J Virol 91(2)
Perin PM, Haid S, Brown RJ, Doerrbecker J, Schulze K, Zeilinger C, von Schaewen M, Heller B, Vercauteren K, Luxenburger E, Baktash YM, Vondran FW, Speerstra S, Awadh A, Mukhtarov F, Schang LM, Kirschning A, Muller R, Guzman CA, Kaderali L, Randall G, Meuleman P, Ploss A, Pietschmann T (2016) Flunarizine prevents hepatitis C virus membrane fusion in a genotype-dependent manner by targeting the potential fusion peptide within E1. Hepatology 63(1): 49-62.
Haid S, Grethe C, Bankwitz D, Grunwald T, Pietschmann T (2015) Identification of a Human Respiratory Syncytial Virus Cell Entry Inhibitor by Using a Novel Lentiviral Pseudotype System. J Virol 90(6): 3065-3073.
Koutsoudakis G, Romero-Brey I, Berger C, Perez-Vilaro G, Monteiro Perin P, Vondran FW, Kalesse M, Harmrolfs K, Muller R, Martinez JP, Pietschmann T, Bartenschlager R, Bronstrup M, Meyerhans A, Diez J (2015) Soraphen A: A broad-spectrum antiviral natural product with potent anti-hepatitis C virus activity. J Hepatol 63(4): 813-821.
Finanzierung:
BMBF: Validierung gezielter Lipid-Adressierung als neue Strategie für antivirale Substanzen mit breitem Wirkspektrum (LABoVIR) – Antivirale und toxikologische Tests
HZI: Pre-4D drug discovery
DZIF, MWK: Rapid identification of repurposing candidates & Discovery and development of broad-spectrum coronavirus antivirals
COFONI: COFONI "Antivirale Strategien: Wirk- und Impfstoffe"
HZI Creativity and Cooperativity Call: Targeting exoribonuclease activity of SARS CoV-2
Host and viral factors that govern susceptibility to HCV and RSV
Diagnostics and therapy for human respiratory syncytial virus (hRSV) EP17195522.2, pending
Carpentier A, Sheldon J, Vondran FWR, Brown RJ, Pietschmann T (2020) Efficient acute and chronic infection of stem cell-derived hepatocytes by hepatitis C virus. Gut 69(9): 1659-1666.
Baier C, Haid S, Beilken A, Behnert A, Wetzke M, Brown RJP, Schmitt C, Ebadi E, Hansen G, Schulz TF, Pietschmann T, Bange FC (2018) Molecular characteristics and successful management of a respiratory syncytial virus outbreak among pediatric patients with hemato-oncological disease. Antimicrob Resist Infect Control 7: 21.
Weller R, Hueging K, Brown RJP, Todt D, Joecks S, Vondran FWR, Pietschmann T (2017) Hepatitis C virus strain-dependent usage of apolipoprotein E modulates assembly efficiency and specific infectivity of secreted virions. J Virol. pii: JVI.00422-17.
Vieyres G, Welsch K, Gerold G, Gentzsch J, Kahl S, Vondran FW, Kaderali L, Pietschmann T (2016) ABHD5/CGI-58, the Chanarin-Dorfman Syndrome Protein, Mobilises Lipid Stores for Hepatitis C Virus Production. PLoS Pathog 12(4): e1005568.
Haid S, Grethe C, Dill MT, Heim M, Kaderali L, Pietschmann T (2014) Isolate-dependent use of claudins for cell entry by hepatitis C virus. Hepatology 59(1): 24-34.
Funding:
VolkswagenStiftung\MWK: INtegrative Data analytIcs for Respiratory syncytiAl virus RIsk Assessment
HZI: Pre-4D Diagnose RSV
RESIST: Resolving Infection Susceptibility