Projects Experimental Infection Research

Immunological sensing of cytomegalovirus (CMV)
Currently 60-100% of the world´s population is latently infected with HCMV. This represents a major health challenge because in immunocompromised individuals virus reactivation can lead to severe morbidity and mortality. Nevertheless, in immunocompetent individuals primary HCMV infection is mostly unnoticed and the virus developed sophisticated means to escape immunity and to persist in a latent state. We aim to better understand how HCMV is sensed by the immune system and how antiviral immune mechanisms are induced. Therefore, we focus especially on sensing of CMV by myeloid cells in the human and murine model and on the induction of type I interferons which can exhibit pro- and anti-viral effects on CMV infection. We discovered that in human monocyte derived myeloid cells sensing of HCMV infections depends on productive infection and is mediated via the cGAS/STING axis (Paijo et al., 2016). More recently we found that during the early phase of MCMV infection IFN-β responses are induced in Kupffer cells within the liver. Notably, under such conditions MCMV is also sensed by the cGAS/STING axis (Tegtmeyer et al., 2019). Interestingly, hepatocyte-derived MCMV does not infect other cells of the body until day 5 of infection, whereas from day 8 on hepatocyte-derived MCMV disseminates to the salivary gland (Tegtmeyer et al., 2019). This dissemination is dependent on the viral component MCK2 and is mediated by MCMV infected myeloid cells. In the future we will in depth analyze which myeloid cell subsets contribute to MCMV dissemination (AFR-DFG German French project).
Recently, we found that monocyte-derived cells only express type I IFN when stimulated by cell-free HCMV, or upon encounter of HCMV-infected cells that already produce cell-free virus. Nevertheless, also in the absence of cell-free virus, i.e., upon co-culture of infected epithelial/endothelial cells with monocyte-derived macrophages (moMΦ) or dendritic cells (moDC), antiviral responses are induced that limit HCMV spread (Becker et al., 2018). This observation together with our recently improved understanding of antigen presentation by human dendritic cells (Döring et al., 2019) is applied to develop improved methods for the generation of HCMV-specific T cell products for the use in patients with severe HCMV infection (Forschergruppe 2830, P04).
By applying single cell sequencing to HCMV infected human dendritic cells we aim to elucidate the delicate balance between the host response and evasion strategies deployed by HCMV. The objective is to better understand how HCMV establishes latent infection in myeloid cells (SFB 900, B2).
Publications
Regulation of acute hepatitis
The pathogenesis of virus-induced hepatitis is not well understood and immunological strategies aiming at re-adjustment of immunological processes within the liver are not available, yet. Recently we studied why upon enterovirus infection very divergent disease courses can be detected, ranging from mild symptoms to severe myocarditis. We found that upon Coxsackievirus B3 (CVB3) infection of mice hepatocytes are key innate effector cells that mount protective type I IFN responses. In conditional mice devoid of type I IFN receptor signaling selectively on hepatocytes, CVB3 infection is not controlled and the virus disseminates similarly as in mice devoid of the type I IFN receptor in all cells (Koestner et al., 2018). Furthermore, we analyzed how liver resident Kupffer cells and infiltrating peripheral myeloid cells react upon hepatic infection (Borst et al., 2018). To this end, we studied vaccina virus as well as MCMV infection of mice. Interestingly, right after infection Kupffer cells disappear from the liver, whereas peripheral myeloid cells infiltrate the liver and later differentiated to cells that are very reminiscent to Kupffer cells. Furthermore, type I IFN receptor signaling of myeloid cells modulates the severity of hepatitis, whereas type I IFN receptor signaling of hepatocytes has no effect, at least in VACV and MCMV induced hepatitis. Currently it is analyzed how infiltrated myeloid cells differentiate to cells that are reminiscent of Kupffer cells within the liver. The long term objective of these studies is to better understand local immune mechanisms within the liver that modulate anti-viral defense and inflammation. It is planned to use these models in order to study new tracers for in vivo imaging. Such data will help to develop new diagnostic tools in the clinics.
Publications
Virus control within the CNS
Even though virus infection of the central nervous system (CNS) is a relatively rare event, it causes high morbidity and mortality. Currently, efficacious therapies are very limited due to an incomplete understanding of molecular processes underlying anti-viral control and pathogenesis within the CNS. Upon intranasal instillation, VSV infects olfactory sensory neurons within the nasal epithelium and then moves within the axons into peripheral areas of the olfactory bulb (Detje et al., 2009). There, primarily astrocytes produce type I IFN that protects against lethal encephalitis (Detje et al., 2015). Between day 3 and 6 after infection microglia gets activated and proliferates in the olfactory bulb and accumulates in peripheral areas to form an innate immune shield. Interestingly, type I IFN receptor signaling of astrocytes and neurons is needed in order to obtain full microglia activation. Upon depletion of microglia, or after partial microglia activation, virus dissemination proceeds, which results in lethal encephalitis (Chhatbar et al., 2018). More recently we discovered that amongst day 6 infiltrating leucocytes CD8+ T cells are critically required to promote survival. We found that redundant RIG-I like receptor (RLR) and Toll-like receptor (TLR) signaling confers the induction of IFN-β responses within the brain, whereas only TLR signaling of astrocytes and neurons is needed to recruit leucocytes to the brain. Currently we are analyzing how herpes simplex virus type I (HSV) is sensed within the CNS and how type I interferon responses trigger protective immunity against this virus (Helmholtz Zukunftsthema “Immunology and Inflammation”).
Viral encephalitis often is associated with acute seizures and epilepsy resulting from hippocampal neurodegeneration. The relative roles of microglia versus monocytes in the development of seizures and epilepsy after viral encephalitis are only incompletely understood. In collaboration with Prof. Löscher, Institute for Pharmacology, Veterinary School Hannover, we are using genetic as well as pharmacological approaches to understand the role of microglia and monocytes in seizure development and hippocampal damage. Our studies show that infiltration of monocytes in the CNS after viral encephalitis is involved in seizure development, but does not seem to contribute to hippocampal damage (Waltl et al., 2018). Interestingly, lack of CCR2 and CX3CR1, two chemokine receptors that regulate the responses of monocytes and microglia, results in prevention of hippocampal damage, but not seizure development. Thus seizure development and hippocampal damage after viral encephalitis are outcomes of complex interactions between CNS cells, including microglia and infiltrating monocytes, and associated CNS inflammation due to encephalitis (Kaeufer et al., 2018).
Publications
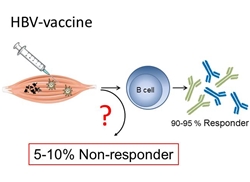
Since 1986, genetically engineered second generation HBV vaccines are on the market which consist the HBV surface antigen (HBsAg). The vaccines are produced in yeast and formulated with alum as adjuvant. They are considered the first true anti-cancer vaccine. Even though second generation HBV vaccines are considered to be amongst the safest and most effective vaccines ever made, approximately 5% of vaccinated individuals do not respond to vaccination. Therefore, we aim at identifying immunological and genetic mechanisms of non-responsiveness to HBV-vaccination. Based on this information we will then search for new strategies to overcome the HBV vaccination non-responsiveness. This project is carried out by an interdisciplinary group of clinicians and immunological experts at different centers of the Helmholtz Association, the German Cancer Research Centre (DKFZ), the Helmholtz-Center Munich, and the HZI, and clinical units of MHH and the Technical University Munich (TUM) (Cluster of Excellence - Resolving Infection Susceptibility (RESIST)).
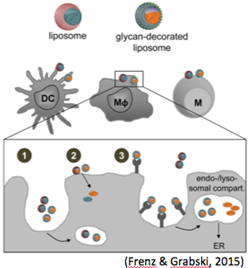
Upon systemic administration of new drugs entirely unexpected adverse effects can be induced in addition to the anticipated effects, which make the development of new compounds very difficult and time consuming. Therefore, in a first step we aim at the development of cell-selective delivery approaches of approved drugs with established functions and known adverse effects. Currently we test different antibiotics encapsulated in glycan-functionalized liposomes (TargoSpheres®) in in vitro and in vivo settings. The objective of these studies is to preferentially deliver antibiotics to alveolar macrophages, which are amongst to most effectively infected cells during early Mycobacterium tuberculosis (Mtb) infection. The hope is that this way antibiotic resistance of Mtb infection can be overcome, adverse effects can be avoided and the overall treatment schedule can be simplified.
More recently we tested the cellular uptake of biodegradable polymeric nanoparticles (NP) made from poly (lactid-co-glycolide) acid (PLGA). Amongst PBMC, antigen presenting cells showed particularly strong PLGA uptake that was even further enhanced when chitosan (CS) coated PLGA was used. This observation points towards the use of CS-PLGA NP as a suitable formulation for vaccines, immunomodulators as well as antibiotics in case of the treatment of pathogens that preferentially infect antigen presenting cells (Duran et al., 2019).
Publications
Towards the development of novel cell therapy options for MSMD patients
Mendelian susceptibility to mycobacterial disease (MSMD) is a genetic disorder that is caused by mutations in the IFN-γ signaling cascade and predisposes patients to severe infections with low pathogenic mycobacteria like the BCG vaccine. Especially patients with autosomal recessive, complete IFN-γR1 deficiency have poor prognosis and often die in early childhood as a result of disseminated infection with BCG and/or other environmental mycobacteria. In cooperation with Dr. Lachmann, Institute of Experimental Hematology at MHH, we are developing novel iPSC based treatment options for MSMD patients.
Publications
Immunological sensing of human cytomegalovirus (HCMV)
Tegtmeyer PK, Spanier J, Borst K, Becker J, Riedl A, Hirche C, Ghita L, Skerra J, Baumann K, Lienenklaus S, Doering M, Ruzsics Z, Kalinke U (2019) STING induces early IFN-β in the liver and constrains myeloid cell-mediated dissemination of murine cytomegalovirus. Nat Commun 10(1): 2830.
Doering M, Blees H, Koller N, Tischer-Zimmermann S, Müsken M, Henrich F, Becker J, Grabski E, Wang J, Janssen H, Zuschratter W, Neefjes J, Klawonn F, Eiz-Vesper B, Tampé R, Kalinke U (2019) Modulation of TAP-dependent antigen compartmentalization during human monocyte-to-DC differentiation. Blood Adv 3(6): 839-850.
Becker J, Kinast V, Doering M, Lipps C, Duran V, Spanier J, Tegtmeyer PK, Wirth D, Cicin-Sain L, Alcamí A, Kalinke U (2018) Human monocyte-derived macrophages inhibit HCMV spread independent of classical antiviral cytokines. Virulence 9(1): 1669-1684.
Hirche C, Frenz T, Haas SF, Doering M, Borst K, Tegtmeyer PK, Brizic I, Jordan S, Keyser K, Chhatbar C, Pronk E, Lin S, Messerle M, Jonjic S, Falk CS, Trumpp A, Essers MAG, Kalinke U (2017) Systemic Virus Infections Differentially Modulate Cell Cycle State and Functionality of Long-Term Hematopoietic Stem Cells In Vivo. Cell Rep 19(11): 2345-2356.
Paijo J, Doering M, Spanier J, Grabski E, Nooruzzaman M, Schmidt T, Witte G, Messerle M, Hornung V, Kaever V, Kalinke U (2016) cGAS Senses Human Cytomegalovirus and Induces Type I Interferon Responses in Human Monocyte-Derived Cells. PLoS Pathog 12(4): e1005546.
Doering M, Lessin I, Frenz T, Spanier J, Kessler A, Tegtmeyer P, Dag F, Thiel N, Trilling M, Lienenklaus S, Weiss S, Scheu S, Messerle M, Cicin-Sain L, Hengel H, Kalinke U (2014) M27 expressed by cytomegalovirus counteracts effective type I interferon induction of myeloid cells but not of plasmacytoid dendritic cells. J Virol 88(23): 13638-13650.
Regulation of acute hepatitis
Koestner W, Spanier J, Klause T, Tegtmeyer PK, Becker J, Herder V, Borst K, Todt D, Lienenklaus S, Gerhauser I, Detje CN, Geffers R, Langereis MA, Vondran FWR, Yuan Q, van Kuppeveld FJM, Ott M, Staeheli P, Steinmann E, Baumgärtner W, Wacker F, Kalinke U (2018) Interferon-beta expression and type I interferon receptor signaling of hepatocytes prevent hepatic necrosis and virus dissemination in Coxsackievirus B3-infected mice. PLoS Pathog 14(8): e1007235.
Borst K, Graalmann T, Kalinke U (2019) Reply to: "Unveiling the depletion of Kupffer cells in experimental hepatocarcinogenesis through liver macrophage subtype-specific markers". Who are you?, new markers needed to discriminate tissue resident Kupffer cells and infiltrating myeloid cells. J Hepatol 71(3): 633-635.
Borst K, Graalmann T, Kalinke U (2019) Reply to: "Lack of Kupffer cell depletion in diethylnitrosamine-induced hepatic inflammation". Stay here or get away, Kupffer cell behavior during liver injury. J Hepatol 70(4): 815-816.
Borst K, Frenz T, Spanier J, Tegtmeyer PK, Chhatbar C, Skerra J, Ghita L, Namineni S, Lienenklaus S, Köster M, Heikenwaelder M, Sutter G, Kalinke U (2018) Type I interferon receptor signaling delays Kupffer cell replenishment during acute fulminant viral hepatitis. J Hepatol 68(4): 682-690.
Pfaender S, Grabski E, Detje CN, Riebesehl N, Lienenklaus S, Steinmann E, Kalinke U, Pietschmann T (2016) Hepatitis C Virus Stimulates Murine CD8alpha-Like Dendritic Cells to Produce Type I Interferon in a TRIF-Dependent Manner. PLoS Pathog 12(7): e1005736.
Grabski E, Wappler I, Pfaender S, Steinmann E, Haid S, Dzionek A, Pietschmann T, Kalinke U (2015) Efficient virus assembly, but not infectivity, determines the magnitude of hepatitis C virus-induced interferon alpha responses of plasmacytoid dendritic cells. J Virol 89(6): 3200-3208.
Virus control within the CNS
Chhatbar C, Detje CN, Grabski E, Borst K, Spanier J, Ghita L, Elliott DA, Jordão MJC, Mueller N, Sutton J, Prajeeth CK, Gudi V, Klein MA, Prinz M, Bradke F, Stangel M, Kalinke U (2018) Type I Interferon Receptor Signaling of Neurons and Astrocytes Regulates Microglia Activation during Viral Encephalitis. Cell Rep 25(1): 118-129.
Kaeufer C, Chhatbar C, Bröer S, Waltl I, Ghita L, Gerhauser I, Kalinke U, Löscher W (2018) Chemokine receptors Ccr2 and Cx3cr1 regulate viral encephalitis-induced hippocampal damage but not seizures. PNAS 115(38): E8929-E8938.
Waltl I, Käufer C, Bröer S, Chhatbar C, Ghita L, Gerhauser I, Anjum M, Kalinke U, Löscher W (2018) Macrophage depletion by liposome-encapsulated clodronate suppresses seizures but not hippocampal damage after acute viral encephalitis. Neurobiol Dis 110: 192-205.
Detje CN, Lienenklaus S, Chhatbar C, Spanier J, Prajeeth CK, Soldner C, Tovey MG, Schluter D, Weiss S, Stangel M, Kalinke U (2015) Upon intranasal vesicular stomatitis virus infection, astrocytes in the olfactory bulb are important interferon Beta producers that protect from lethal encephalitis. J Virol 89(5): 2731-2738.
Detje CN, Meyer T, Schmidt H, Kreuz D, Rose JK, Bechmann I, Prinz M, Kalinke U (2009) Local type I IFN receptor signaling protects against virus spread within the central nervous system. J Immunol 182(4): 2297-2304.
Cell-selective delivery of active compounds
Durán V*, Yasar H*, Becker J, Thiyagarajan D, Loretz B, Kalinke U*, Lehr CM* (2019) Preferential uptake of chitosan-coated PLGA nanoparticles by primary human antigen presenting cells. Nanomedicine 21: 102073. * shared first and last authorship
Frenz T, Grabski E, Duran V, Hozsa C, Stepczynska A, Furch M, Gieseler RK, Kalinke U (2015) Antigen presenting cell-selective drug delivery by glycan-decorated nanocarriers. Eur J Pharm Biopharm 95(Pt A): 13-17.
Towards the development of novel cell therapy options for MSMD patients
Neehus AL, Lam J, Haake K, Merkert S, Schmidt N, Mucci A, Ackermann M, Schubert M, Happle C, Kühnel MP, Blank P, Philipp F, Goethe R, Jonigk D, Martin U, Kalinke U, Baumann U, Schambach A, Roesler J, Lachmann N (2018) Impaired IFNγ-Signaling and Mycobacterial Clearance in IFNγR1-Deficient Human iPSC-Derived Macrophages. Stem Cell Reports 10(1): 7-16.
Hetzel M, Mucci A, Blank P, Nguyen AHH, Schiller J, Halle O, Kühnel MP, Billig S, Meineke R, Brand D, Herder V, Baumgärtner W, Bange FC, Goethe R, Jonigk D, Förster R, Gentner B, Casanova JL, Bustamante J, Schambach A, Kalinke U, Lachmann N (2018) Hematopoietic stem cell gene therapy for IFNγR1 deficiency protects mice from mycobacterial infections. Blood 131(5): 533-545.
